New synthetic methods for the chemistry/biology interface

Team Composition
Permanent Staff
Erica. Benedetti, CRCN, HDR
Dylan, Coelho, IR
Anton Granzhan, CRCN, HDR
Riccardo. Piccardi, CRCN
PhD Students
Jiayi Zhu
Brian Deboutière
Camille Richagneux
Post-Doc
Joseph Boissieras
Rongyu Sun
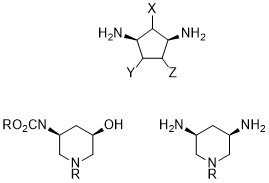
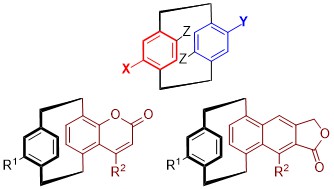
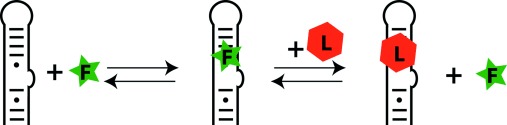
RNA ligands: chemical spaces, probes and analytical methods
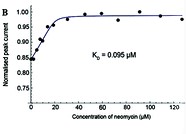
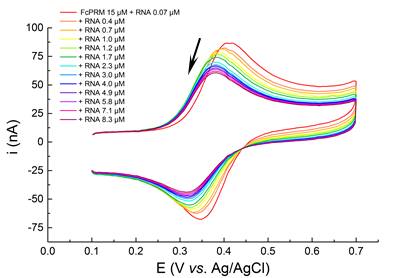
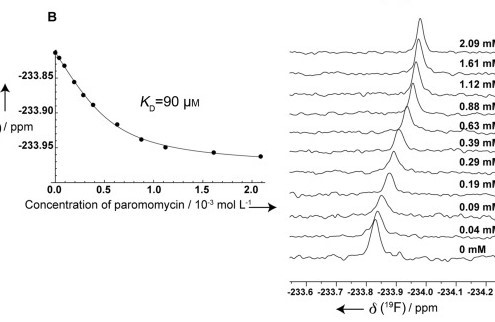
Main publications
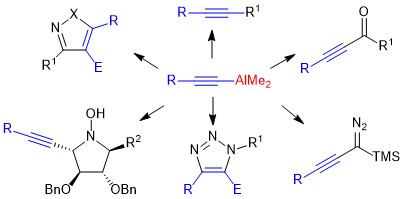
New synthetic methods for the chemistry/biology interface
Team composition

Group Leader
Permanent Staff
Erica. Benedetti, CRCN, HDR
Dylan, Coelho, IR
Anton Granzhan, CRCN, HDR
Riccardo. Piccardi, CRCN
PhD Students
Jiayi Zhu
Brian Deboutière
Camille Richagneux
Post-doc
Joseph Boissieras
Rongyu Sun
Team Description
The interests of the group span a wide range of topics in the fields of synthetic organic chemistry and chemical biology, all of them being grounded on molecular innovation. Our works involve the exploration of new chemical spaces as well as the development of synthetic tools or analytical methods for studying and / or interfering with biological regulatory mechanisms. Our research activities are organized around two main axes:
RNA ligands: chemical spaces, probes and analytical methods