Synthesis and reactivity of organoaluminum reagents
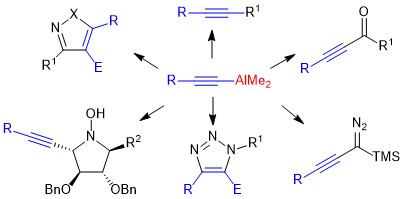
Organoaluminum compounds are still much less used in organic synthesis than classical organometallic reagents (organolithium, magnesium, zinc, copper compounds) The high Lewis acidity of these reagents, combined with their low nucleophilicity, nevertheless makes them potentially powerful tools for selective transformations. We are particularly interested in the reactivity of dialkylaluminum acetylides, prepared according to an original synthetic pathway developed in the laboratory.
Main publications
1. R. Piccardi, S. Turcaud, E. Benedetti, L. Micouin Synthesis and Reactivity of Mixed Dimethylalkynylaluminum Reagents. Synthesis 2019, 51, 97-106
2. T. Zhao, R. Piccardi, L. Micouin, Rapid and Effective Synthesis of α-Acyloxy-α-alkynyltrimethylsilanes, Org. Lett. 2018 20, 5015-5018.
3. R. Piccardi, A. Coffinet, E. Benedetti, S. Turcaud, L. Micouin, Continuous Flow Synthesis of Dimethylalkynylaluminum Reagents, Synthesis 2016, 48, 3272-3278.
4. O. Jackowski, T. Lecourt, L. Micouin Direct Synthesis of Polysubstituted Aluminoisoxazoles and Pyrazoles by a Metalative Cyclization. Org. Lett., 2011, 13, 5664–5667
5. Y. Zhou, T. Lecourt, L. Micouin, Direct Synthesis of 1,4-Disubstituted-5-alumino-1,2,3-triazoles By a Copper-Catalyzed Cycloaddition of Organic Azides and Mixed-Aluminum acetylides. Angew. Chem. Int. Ed. 2010, 49, 2607-2610.
