Chemistry towards life sciences

Team composition
Team Leaders
C. Gravier Pelletier
M. Etheve Quelquejeu
Thematic Groups
Organics synthesis for biomedical research

C. GRAVIER-PELLETIER, DR
F. Berhal, MCU
M. Bosco, IE
P. Busca, MCU, HDR
S. Calvet-Vitale, MCU
L. Le Corre, IE
G. Prestat, PR
M. Seremes, Tech
T. Xavier, MCU
H. Esteves, Ph.D.
J. Feng, Ph.D.
G. Lee, Ph.D.
H. Wan, Ph.D.
Chemistry of RNAs, Nucleosides, Peptides and Heterocycles

M. ETHEVE-QUELQUEJEU, PR
D. Bouquet, IE
E. Braud, MCU, HDR
H. Chen, MCU, HDR
C. Garbay, PRem
L. Iannazzo, CR
Y. Afandizada, Ph.D.
Yan Badji, Ph.D.
K. Burke, Ph.D.
D. Coelho, Post-Doc
Y. Colas, Post-Doc
D. Jianxun, Ph.D.
L. Mossino, Ph.D.
The team gathers four thematic groups with common research interests in the field of molecular and supramolecular chemistry to study, mimic or interact with living systems. Major topics include:
Methodological developments
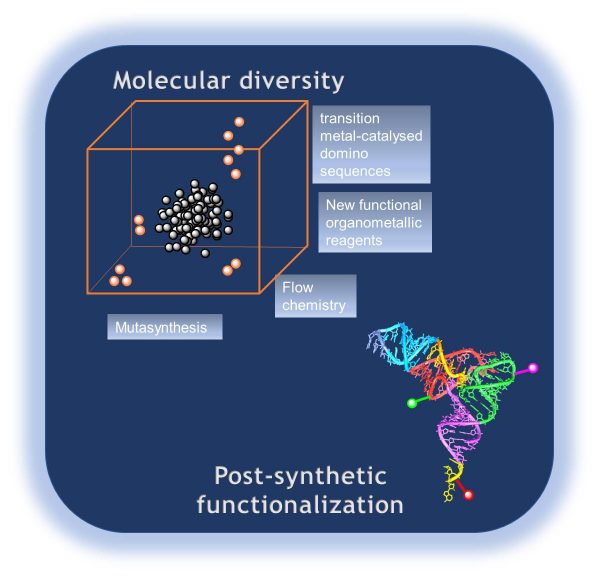
This axis addresses two major issues: the need for greater chemical diversity and the need for new methodologies allowing the selective functionalization of complex biomolecules.
The first approach aims at developing complexity-oriented synthetic methods by: 1) setting up short and scalable routes to complex or densely functionalized scaffolds notably by transition metal-catalyzed domino sequences and post-(de)functionalization. 2) Generating novel chemical entities by exploring the synthetic potential of organoaluminum reagents. 3) Exploring the chemical space between natural products and synthetic compounds using engineered microorganisms to produce natural-product like new chemical entities in a mutasynthetic approach. And finally 4) widen the chemical space of reactions conducted under kinetic control through flow-chemistry technics.
The second approach concerns biomolecules with the development of post-synthetic methodologies for bi-functionalization of RNAs with reactive groups, affinity purification tags, and fluorescent probes.
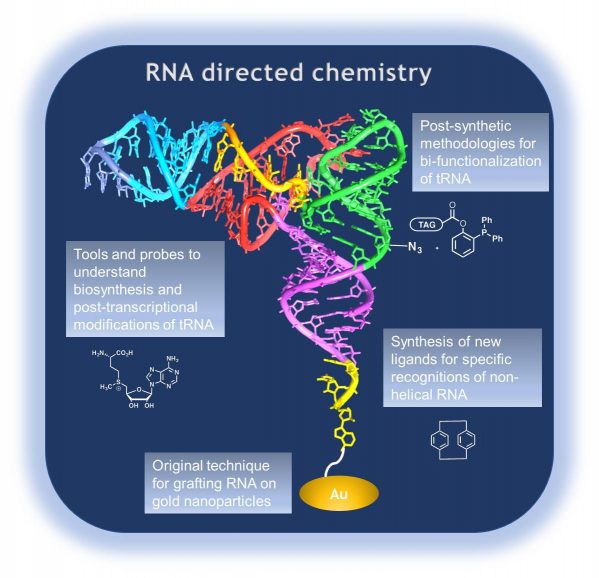
RNA directed chemistry
Investigating the biology of RNA is of great interest to better explore living systems at a molecular/supramolecular level. Our goals are to develop: 1) Tools and probes to better understand the biosynthesis and post-transcriptional modifications of RNA by designing analogues of S-adenosyl-L-methionine (SAM), an ubiquitous cofactor acting as a carbon source for methylation or more complex post-transcriptional modifications. We notably focus on RNA methylation by methyltransferases such as TrmK, a bacterial tRNA MTase. And 2) Molecular probes for sensing single-stranded RNA regions. The [2.2]paracyclophane scaffold that cannot intercalate in classical multi-stranded helices is used for the design of new ligands allowing the specific recognition of non-helical RNA tridimensional motifs through either a fluorimetric response or SERS-based detection (after grafting on gold surfaces). These sensors will be used as reporters to drive the design of small molecules capable of binding to RNA and interfering with its biological activity.
 |
 |
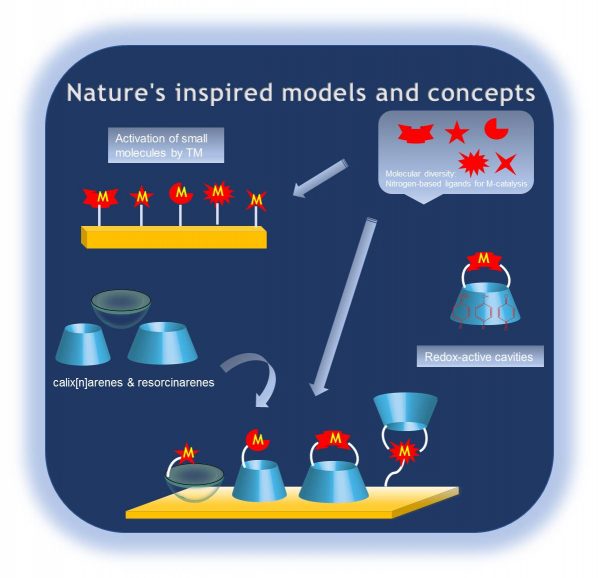
Nature’s inspired models and concepts
Biomimetic coordination chemistry is a major field devoted to a better understanding of fundamental enzymatic processes. It comprises molecular recognition on the one hand and reactivity and catalysis on the other hand. Our major goals are to: 1) Elaborate efficient and selective receptors for small biological molecules. These are based on the host-guest chemistry of cavity-complexes with the aim to provide probes (either water-soluble or electrode-grafted) with high affinity constants and allowing spectroscopic reporting in a biological context. 2) Obtain new fundamental insights into electron and proton coupled redox processes under the supramolecular control provided by the cavities surrounding the metal ion. This is mainly focused on redox-active cavities (integrating quinone or phenol moieties), which, up to now, have never been exploited in the context of biomimetic coordination chemistry. And 3) Elaborate new catalysts for the activation of small molecules (O2, N2, CO2, H2…). Two levels of studies will be conducted: the first one concerns solution studies of the new complexes, whereas the second one is based on the grafting of the ligands/cavities/metal complexes on electro-active surfaces.
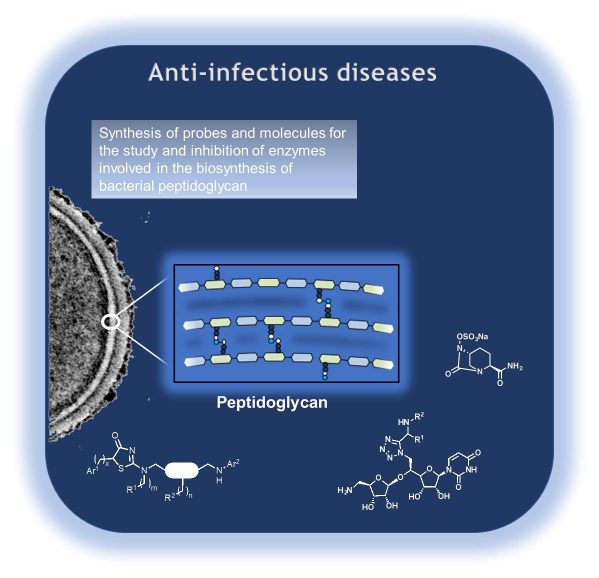
Targets and Drug design
Developing drug design programs and molecular tools for the study of biological targets is also a major priority. We are focusing on: 1) Infectious diseases. The goals are to study and inhibit enzymes involved in the biosynthesis of bacterial peptidoglycan, with different approaches: i) the use of functionalized RNAs, to study the tRNA recognition by amino-acyltransferases of the Fem family. ii) the design and synthesis of inhibitors to study LDts-transpeptidases and MurT/GatD amidotransferase complex. iii) The discovery of new MraY inhibitors through a combination of virtual screening and multi-component synthesis. 2) New antiviral therapy. The goal is to use novel classes of compounds we developed, and able to interfere with the proliferation of the HIV, to discover and study new targets in its replication cycle. 3) Congenital diseases. Novel fluorescent and biotinylated probes are developed to assay, purify and inhibit mammalian Dolichol Linked Oligosaccharide DiPhosphatases (DLODPs) to explore their roles in rare inherited diseases. 4) Cancer. Focal adhesion kinase (FAK) belongs to the most attractive tyrosine kinase targets for cancer chemotherapy. We develop new potent irreversible inhibitors of FAK based on the first crystallographic structure of a covalent complex between FAK and an irreversible inhibitor we recently obtained.
 |
 |
Main Publications
Chemistry towards life sciences
TEAM COMPOSITION
Organics synthesis for biomedical research
C. Gravier-Pelletier, DR
F. Berhal, MCU
P. Busca, MCU, HDR
S. Calvet-Vitale, MCU
L. Le Corre, IGE
G. Prestat, PR
Y. Bourne-Branchu, ATER
M. Bosco, Post-Doc
H. Gao, Post-Doc
A. Corio, Ph.D.
G. Kirby, Ph.D.
M. Oliver, Ph.D.
H. Wan, Ph.D.
RNA, nucleosides, peptides and heterocyclic chemistry
M. Etheve-Quelquejeu, PR
E. Braud, MCU, HDR
H. Chen, MCU, HDR
C. Garbay, PRem
L. Iannazzo, CR
K. Bartosik, Post-Doc
D. Coelho, Ph.D.
F. Bouchet, Ph.D.
C. Kitoun, Ph.D.
S. Saidjalolov, Ph.D.
New synthetic methods for the chemistry/biology interface
L. Micouin, DR
E. Benedetti, CR
R. Piccardi, CR
O. Christin, Post-Doc
R. Sun, Post-Doc
J. Zhu, Ph.D.
Research
Methodological developments
This axis addresses two major issues: the need for greater chemical diversity and the need for new methodologies allowing the selective functionalization of complex biomolecules.
The first approach aims at developing complexity-oriented synthetic methods by: 1) setting up short and scalable routes to complex or densely functionalized scaffolds notably by transition metal-catalyzed domino sequences and post-(de)functionalization. 2) Generating novel chemical entities by exploring the synthetic potential of organoaluminum reagents. 3) Exploring the chemical space between natural products and synthetic compounds using engineered microorganisms to produce natural-product like new chemical entities in a mutasynthetic approach. And finally 4) widen the chemical space of reactions conducted under kinetic control through flow-chemistry technics.
The second approach concerns biomolecules with the development of post-synthetic methodologies for bi-functionalization of RNAs with reactive groups, affinity purification tags, and fluorescent probes.

Targets and Drug design
Developing drug design programs and molecular tools for the study of biological targets is also a major priority. We are focusing on: 1) Infectious diseases. The goals are to study and inhibit enzymes involved in the biosynthesis of bacterial peptidoglycan, with different approaches: i) the use of functionalized RNAs, to study the tRNA recognition by amino-acyltransferases of the Fem family. ii) the design and synthesis of inhibitors to study LDts-transpeptidases and MurT/GatD amidotransferase complex. iii) The discovery of new MraY inhibitors through a combination of virtual screening and multi-component synthesis. 2) New antiviral therapy. The goal is to use novel classes of compounds we developed, and able to interfere with the proliferation of the HIV, to discover and study new targets in its replication cycle. 3) Congenital diseases. Novel fluorescent and biotinylated probes are developed to assay, purify and inhibit mammalian Dolichol Linked Oligosaccharide DiPhosphatases (DLODPs) to explore their roles in rare inherited diseases. 4) Cancer. Focal adhesion kinase (FAK) belongs to the most attractive tyrosine kinase targets for cancer chemotherapy. We develop new potent irreversible inhibitors of FAK based on the first crystallographic structure of a covalent complex between FAK and an irreversible inhibitor we recently obtained.

RNA directed chemistry
Investigating the biology of RNA is of great interest to better explore living systems at a molecular/supramolecular level. Our goals are to develop: 1) Tools and probes to better understand the biosynthesis and post-transcriptional modifications of RNA by designing analogues of S-adenosyl-L-methionine (SAM), an ubiquitous cofactor acting as a carbon source for methylation or more complex post-transcriptional modifications. We notably focus on RNA methylation by methyltransferases such as TrmK, a bacterial tRNA MTase. And 2) Molecular probes for sensing single-stranded RNA regions. The [2.2]paracyclophane scaffold that cannot intercalate in classical multi-stranded helices is used for the design of new ligands allowing the specific recognition of non-helical RNA tridimensional motifs through either a fluorimetric response or SERS-based detection (after grafting on gold surfaces). These sensors will be used as reporters to drive the design of small molecules capable of binding to RNA and interfering with its biological activity.

Nature’s inspired models and concepts
Biomimetic coordination chemistry is a major field devoted to a better understanding of fundamental enzymatic processes. It comprises molecular recognition on the one hand and reactivity and catalysis on the other hand. Our major goals are to: 1) Elaborate efficient and selective receptors for small biological molecules. These are based on the host-guest chemistry of cavity-complexes with the aim to provide probes (either water-soluble or electrode-grafted) with high affinity constants and allowing spectroscopic reporting in a biological context. 2) Obtain new fundamental insights into electron and proton coupled redox processes under the supramolecular control provided by the cavities surrounding the metal ion. This is mainly focused on redox-active cavities (integrating quinone or phenol moieties), which, up to now, have never been exploited in the context of biomimetic coordination chemistry. And 3) Elaborate new catalysts for the activation of small molecules (O2, N2, CO2, H2…). Two levels of studies will be conducted: the first one concerns solution studies of the new complexes, whereas the second one is based on the grafting of the ligands/cavities/metal complexes on electro-active surfaces.



