Chemistry of RNAs, Nucleosides, Peptides and Heterocycles
Team Composition
Permanent Staff
E. Braud, Pr
H. Chen, MCU, HDR
C. Garbay, PRem
L. Iannazzo, CR, HDR
L. Le Corre, IE
Non Permanent Staff
Du Jianxun, Post-Doc
Yoann Colas, Post-Doc
Clara Testard, Post-Doc
PhD Students
Yan Badji
Katie Burke
Former Members
D. Coelho, Post-doc
Y. Afandizada
L. Mossino
T. Xavier, Post-doc
F. Bouchet
S. Saidjalolov
C. Kitoun
K. Bartosik, Post-Doc
S. Leprevost, ASI
B. Li
Chemistry of RNAs :
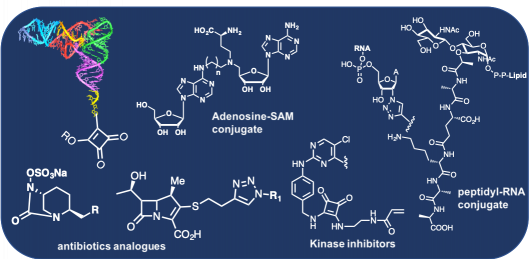
Development of new methodologies for post-functionalization of RNAs. Using our methodologies, we were able to synthesized :
– Peptidyl-RNA conjugates for the study of FmhB of Staphyloccocus Aureus
– Stable aminoacyl-tRNA analogues to explore non ribosomal peptide synthesis processes.
– RNA-SAM cofactor conjugates to study m6A-RNA methyltransferases.
– Development of Nanospectroscopy for mapping RNA profile in Tumor-educated blood platelets.
Chemistry of Peptides
We synthezise peptidoglycan fragments to study L,D- transpeptidases enzymes involved in cell wall synthesis of Mycobacterium Tuberculosis.
Chemistry of Heterocycles
Development of new chemistry for the synthesis of carbapenem or avibactam analogues in order to inhibit or study the cell wall synthesis of bacteria. Development of selective inhibitors of FAK kinase is also one of our current projects.
All of these projects are at the interface between chemistry and biology and benefit from collaboration at the local, national and international levels.
1. Y. S. Polikanov, M. Etheve-Quelquejeu, R. Micura, “Synthesis of Peptidyl-tRNA Mimics for Structural Biology Applications”Accounts of Chemical Research, 2023, doi-org.ezproxy.u-paris.fr/10.1021
2. L. Iannazzo, E. Braud, C. Atdjian, M. Ethève-Quelquejeu & al., Synthesis of RNA-cofactor conjugates and structural exploration of RNA recognition by an m6A RNA methyltransferase, Nucleic Acids Research, 2022, gkac354, https://doi.org/10.1093/nar/gkac354
3. S. Saidjalolov, E. Braud, L. Iannazzo, M. Etheve-Quelquejeu & al., Click and Release Chemistry for Activity-based Purification of b-lactam targets, Chem. Eur. J., 2021, DOI:10.1002/chem.202100653
4. C. Kitoun, M. Etheve-Quelquejeu, L. Iannazzo & al. Phosphine-Mediated Bioconjugation of 3’-end of RNA, Org. Lett. 2020, DOI:10.1021/acs.orglett.0c02982.
5. F. Bouchet, H. Atze & al. Diazabicyclooctane functionalization for inhibition of β-lactamases from enterobacteria, Journal of Medicinal Chemistry, 2020, 63, 10, 5257–5273 https://doi.org/10.1021/acs.jmedchem.9b02125
6. C. Atdjian, L. Iannazzo, E. Braud & al. Synthesis of SAM-adenosine conjugates for the study of m6A-RNA methyltransferases Eur. J. Org. Chem., 2018, 4411-4425.
Chemistry of RNAs, Nucleosides, Peptides and Heterocycles
Team composition
Permanent Staff
Permanent Staff
E. Braud, Pr
H. Chen, MCU, HDR
C. Garbay, PRem
L. Iannazzo, CR, HDR
L. Le Corre, IE
Non Permanent Staff
Du Jianxun, Post-Doc
Yoann Colas, Post-Doc
Clara Testard, Post-Doc
PhD Students
Yan Badji
Katie Burke
Former Members
D. Coelho, Post-doc
Y. Afandizada
L. Mossino
T. Xavier, Post-doc
F. Bouchet
S. Saidjalolov
C. Kitoun
K. Bartosik, Post-Doc
S. Leprevost, ASI
B. Li
Chemistry of RNAs :
Development of new methodologies for post-functionalization of RNAs. Using our methodologies, we were able to synthesized :
– Peptidyl-RNA conjugates for the study of FmhB of Staphyloccocus Aureus
– Stable aminoacyl-tRNA analogues to explore non ribosomal peptide synthesis processes.
– RNA-SAM cofactor conjugates to study m6A-RNA methyltransferases.
– Development of Nanospectroscopy for mapping RNA profile in Tumor-educated blood platelets.

Chemistry of Peptides
We synthezise peptidoglycan fragments to study L,D- transpeptidases enzymes involved in cell wall synthesis of Mycobacterium Tuberculosis.
Chemistry of Heterocycles
Development of new chemistry for the synthesis of carbapenem or avibactam analogues in order to inhibit or study the cell wall synthesis of bacteria. Development of selective inhibitors of FAK kinase is also one of our current projects.
All of these projects are at the interface between chemistry and biology and benefit from collaboration at the local, national and international levels.
1. Y. S. Polikanov, M. Etheve-Quelquejeu, R. Micura, “Synthesis of Peptidyl-tRNA Mimics for Structural Biology Applications”Accounts of Chemical Research, 2023, doi-org.ezproxy.u-paris.fr/10.1021
2. L. Iannazzo, E. Braud, C. Atdjian, M. Ethève-Quelquejeu & al., Synthesis of RNA-cofactor conjugates and structural exploration of RNA recognition by an m6A RNA methyltransferase, Nucleic Acids Research, 2022, gkac354, https://doi.org/10.1093/nar/gkac354
3. S. Saidjalolov, E. Braud, L. Iannazzo, M. Etheve-Quelquejeu & al., Click and Release Chemistry for Activity-based Purification of b-lactam targets, Chem. Eur. J., 2021, DOI:10.1002/chem.202100653
4. C. Kitoun, M. Etheve-Quelquejeu, L. Iannazzo & al. Phosphine-Mediated Bioconjugation of 3’-end of RNA, Org. Lett. 2020, DOI:10.1021/acs.orglett.0c02982.
5. F. Bouchet, H. Atze & al. Diazabicyclooctane functionalization for inhibition of β-lactamases from enterobacteria, Journal of Medicinal Chemistry, 2020, 63, 10, 5257–5273 https://doi.org/10.1021/acs.jmedchem.9b02125
6. C. Atdjian, L. Iannazzo, E. Braud & al. Synthesis of SAM-adenosine conjugates for the study of m6A-RNA methyltransferases Eur. J. Org. Chem., 2018, 4411-4425.


