Chiral paracyclophanes
The selective functionalization of [2.2] paracyclophanes allows the exploration of new chemical spaces and new physicochemical properties of three-dimensional chiral aromatic compounds (optical properties, stereoelectronic properties, catalytic activities …). We have recently developed a very simple, scalable pathway for the preparation of enantiopure paracyclophane compounds. These starting material can lead to various chiral 3D extended aromatic systems with peculiar optical properties. Our team is also interested in the diversity oriented synthesis of paracyclophane fragments for the design of single stranded RNA binders.
Main publications
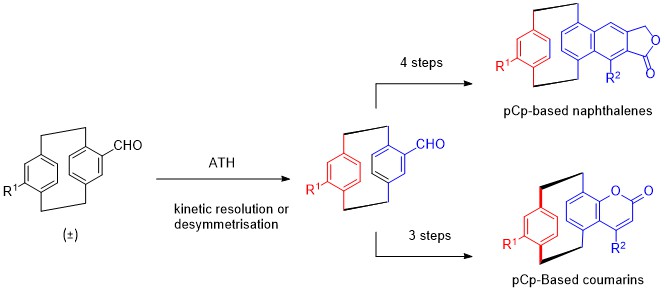
1. M.-L. Delcourt, S. Turcaud, E. Benedetti, L. Micouin Efficient and scalable kinetic resolution of racemic 4-formyl[2,2]paracyclophane via asymmetric transfer hydrogenation, , Adv. Synth. Catal. 2016, 358, 1213-1218.
2. E. Benedetti, M.-L. Delcourt, B. Gatin-Fraudet, S. Turcaud, L. Micouin, Synthesis and photophysical studies of through-space conjugated [2.2]paracyclophane-based naphthalene fluorophores, RSC Advances 2017, 7, 50472-50476
3. M.-L. Delcourt, S. Felder, E. Benedetti, L. Micouin, Highly Enantioselective Desymmetrization of Centrosymmetric pseudo–para-Diformyl[2.2]paracyclophane via Asymmetric Transfer Hydrogenation, ACS Catalysis 2018 8, 6612-6616.
4. M.-L Delcourt, C. Reynaud, S. Turcaud, L. Favereau, J. Crassous, L. Micouin, E. Benedetti, 3D Coumarin Systems Based on [2.2]Paracyclophane: Synthesis, Spectroscopic Characterization, and Chiroptical Properties. J. Org. Chem. 2019, 84, 888-899.
5. M.-L. Delcourt, S. Felder, S. Turcaud, C. H. Pollok, C. Merten, L. Micouin, E. Benedetti, Highly Enantioselective Asymmetric Transfer Hydrogenation: A Practical and Scalable Method To Efficiently Access Planar Chiral [2.2]Paracyclophanes. J. Org. Chem. 2019, 84, 5369-5382
