Diversity-oriented-synthesis of cyclic polyamines
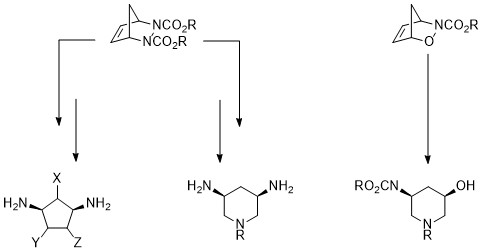
Our team is interested in the asymmetric synthesis of cyclic polyamines. In particular, we focus our attention on the preparation of five- or six-membered rings containing 1,3-diamine motifs for the following reasons:
- Cyclic 1,3-diamines are found in a large amount of molecules possessing interesting biological activities, such as, for example, the ability to interfere with kinases, glycosidases, CNS receptors and various enzymes
- Differently substituted polyamines can be considered as versatile water-soluble building blocks for the design of active substances by a fragment-based approach
- Polyamines can serve as ligands for organometallic complexes.
Main publications
1. P. Gordillo, P. Clerici, L. Micouin, L. Modular Access to N-subtituted cis 5-amino-3-hydroxypiperidines J. Org. Chem. 2017, 82, 7689-7694
2. L. Palais, C. Bournaud, L. Micouin, A. Alexakis, Chem. Eur. J. 2010, 16, 2567-2573.
3. A. Blond, P. Dockerty, R. Álvarez, S. Turcaud, T. Lecourt, L. Micouin, J. Org. Chem. 2013, 78, 12236-12242
4. C. Bournaud, F. Chung, A. Perez Luna, M. Pasco, G. Errasti, T. Lecourt, Micouin Synthesis 2009, 869-887.
5. C. Bournaud, M. Bonin, L. Micouin, Org. Lett. 2006, 8, 3041.
6. C. Bournaud, C. Falciola, T. Lecourt, S. Rosset, A. Alexakis, L. Micouin L. Org. Lett. 2006, 8, 3581.
