You can find in this page the photos which are exhibited in the corridors of our laboratory.
Please feel free to check the text related to each photo to know more about the experience, the authors and the team.
We will let you know soon when you can vote for your 3 favorite photos. Each photo is labelled with a letter (from A to M). The vote is only open for the members of the UMR8601.
The Committee of the QVT project
Rodolphe Alves de Sousa
Sonia Lajnef
Laurent Le Corre
Afaf Mikou
Mandy Sitbon
Accelerating Science: From virtual to reality

Photo taken by Alexandre Cabayé and edited by Floriane Eshak, both molecular modelling PhD students in Francine Acher’s team.
Chemistry operated by Isabelle McCort & Théophile Lambert
When advances in computer sciences influence medicinal chemistry research.
The computer is a hardware dedicated to high-performance computing, it was assembled by our team with an integrated water-cooling system in order to handle long and demanding tasks.
We can see here how this hardware allowed us to validate a hit by using a virtual approach.
This hit targets a transporter that was shown to be involved in a rare genetic disease, the Salla disease.
Once a hit is found virtually, the chemists take over by synthesizing the molecule and characterizing it by NMR.
Our work led to a publication in 2020, while also making the cover of the Journal of Medicinal Chemistry. (Dubois et al., J. Med. Chem., 2020)
This picture is a strong proof of the importance of interdisciplinarity in research, the ability of the virtual to push forward and accelerate the real in order the find faster and more reliable solutions.
Complexes of Fe(II) presenting a hexadentate ligand with pyridine, amino and thiolato coordinating arms. The difference in color is due to the 6th ligand: Cl-, MeCN or CN-.
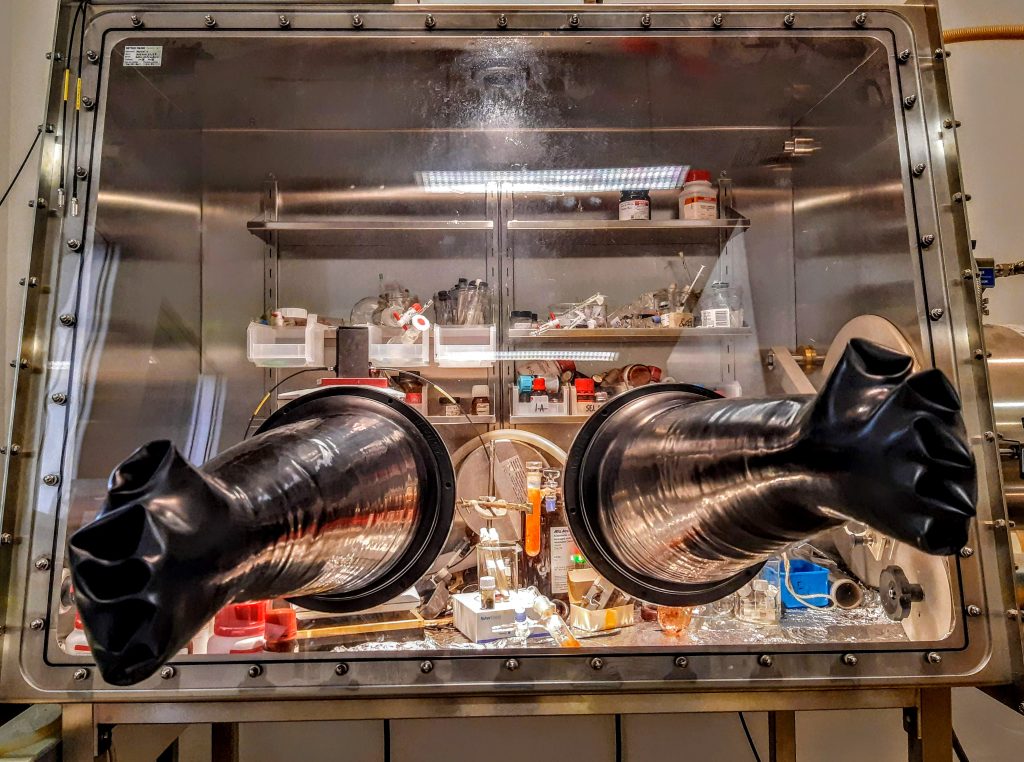
Photo taken by Diana Over
Hydrogen sulfide is not only a very smelly gas, it is also very toxic. However, it turns out that H2S is involved in cell signaling and this involves the formation of persulfides, which are very unstable.
In a biological environment, metal centers in metalloproteins can potentially react with these persulfides and despite the knowledge of these systems, there are very few studies of persulfides as ligands for transition metals. Therefore, in the team “Redox Homeostasis and Bioinorganic Chemistry of Reactive Sulfur Species” we are preparing iron complexes with new ligands, presenting sulfur coordination sites, initially SH groups, which are then transformed into SSH. This photo shows iron complexes presenting a thiolate ligand prepared by Diana Over, Assistant Professor specialized in coordination chemistry .These compounds are intensely colored in orange, red or green due to charge transfer bands and as they are very sensitive to air, they are prepared in the glove box.
“La vie en rose”.

Photo taken by Antoine Maruani
Oxidation reactions are ubiquitous. In organic chemistry, colour change can be a good indication of such reactions and can also make the chemist see life… en rose.
Antoine Maruani
Researcher – Chaire d’Excellence
https://lcbpt.biomedicale.parisdescartes.fr/chemical-biology-immunology/
Dr Antoine Maruani obtained his Master’s degree in Chemistry from École Normale Supérieure of Lyon (France) in 2011. He then joined Prof. Stephen Caddick’s group at University College London (UK) where he obtained his Ph.D. in 2016. Following his experience as a postdoctoral researcher under the supervision of Prof. Vijay Chudasama, he started his independent research on a Ramsay Fellowship (2018–2020) focusing on site-selective modification of native proteins. He was then awarded a Chaire d’Excellence at Paris University (2020–2022) and joined Dr Dalko’s laboratory where he is currently conducting his research on the targeted delivery of drugs using nanomaterials. In 2021, he was appointed as a CNRS Research Scientist.
This photo shows the final step in the preparation of a type of molecule called tetrazine. It is a benzene-like molecule where four C–H units are replaced by nitrogen atoms. The first report of such molecules dates back to the end of the 19th century but very little was known about their properties at that time. Over the years, there have been more and more scientists interested in the tetrazines chemistry. Among their many uses, tetrazine can be reacted with chemical partners with an exquisite selectivity to generate stable conjugates and, maybe more importantly, these reactions can be performed in water or other aqueous media in the presence of biomolecules such as proteins. These are called bioorthogonal reactions as they can occur inside of living systems without interfering with native biochemical processes.
It is this stability and reactivity that we take full advantage of in our research. By attaching a tetrazine at the surface of a very small particle – a nanoparticle, a host of molecules (e.g. fluorophore) or biomolecules (e.g. antibody) can be used to functionalise it rapidly and efficiently both in vitro and in vivo. This opens the doors to a host of applications ranging from drug delivery for cancer treatments to the study of mechanism in the brain, vaccine, imaging…
In the lab, we are currently focusing on utilising this approach to improve the delivery of toxic drugs within a nanoparticle selectively to specific cancer tissues to avoid off-site toxicity and hence minimise potential side-effects.
Frise des matériaux : de l’échelle nanométrique à l’échelle macroscopique.
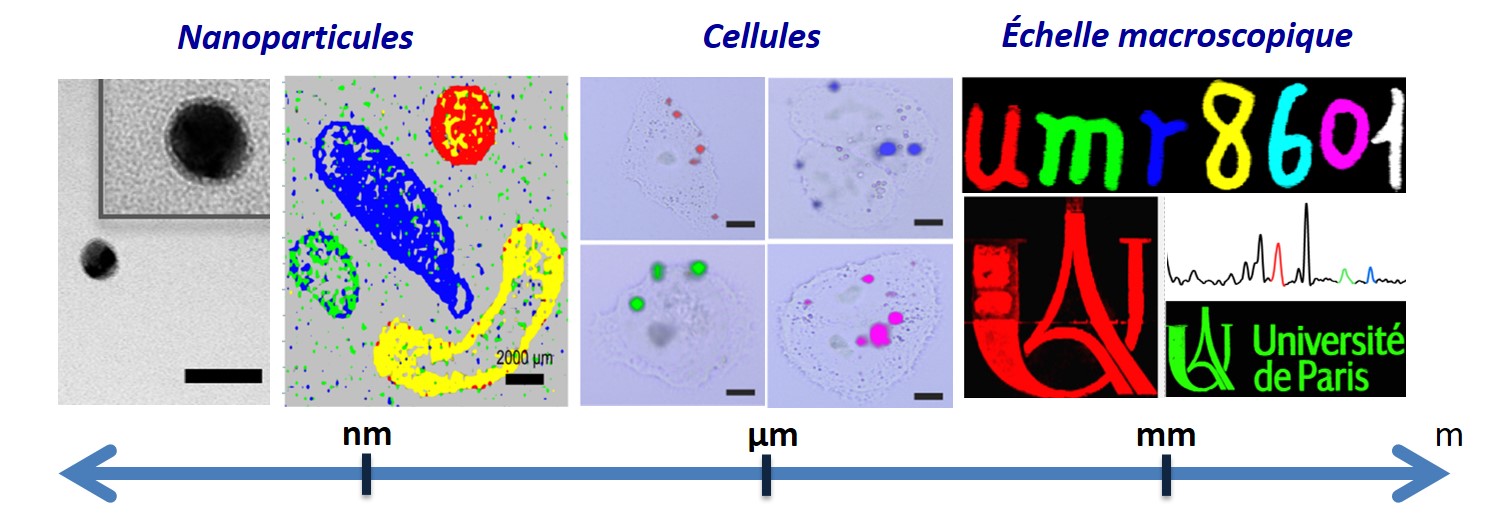
Photo taken by Claire Mangeney
Thanks to their outstanding properties, nanomaterials open up promising prospects for cellular imaging or anticounterfeiting applications.
This image depicts various scales of materials, from an individual silver nanoparticle observed by transmission electron microscopy (TEM) to the deposition of drops of gold nanoparticles on glass or their incorporation within cells and their observation by Raman imaging. Thanks to their outstanding optical properties, silver and gold nanoparticles coated by specific tags exhibit various colors in Raman imaging and can be used as innovative encoded nanotools for bioimaging or as Raman inks for anticounterfeiting applications.
This image gathers various results from the team Nano Biospectroscopy, particularly here from Da Li (PhD), Yun Luo, Philippe Nizard, Delphine Onidas and Claire Mangeney. Their research activities are at the interface between nanoscience, surface chemistry and biology. The team focusses on the design and functionalization of nanoscale tools acting as ideal interfaces to probe biological systems, at different scales.
Detritylation in progress

Photo taken by Clara TESTARD & Laura IANNAZZO
The team « Chemistry of RNAs, nucleosides, peptides et heterocycles » directed by the Pr M. ETHEVE-QUELQUEJEU is specialized, among others, on the development of RNA post-functionalization methodologies. We synthesized modified RNAs and used them as molecular tools to study RNA-dependent enzymes (Fem transferases, Methyltransferases). Our approach is based on the synthesis of modified RNAs by automatic solid phase synthesis (SPS) with a K&A oligonucleotide synthesizer. The picture illustrates one of these steps: the removal of the dimethyoxytrityle protecting group (DMTr) in presence of a trichloroacetic acid solution. During this step, a tertiary carbocation with a strong orange color is generated which help to follow the synthesis of the RNA on the tubing of the machine.
Clara TESTARD obtained her double diploma in chemistry and biology in 2018 at Sorbonne University and then joined the department of chemistry of Ecole Normale Supérieure (ENS). She is currently a Master 2 student at Chimie Paris-Centre (PSL–SU) and joined the team « Chemistry of RNAs, nucleosides, peptides et heterocycles » for her internship. She concentrates on the development of post-functionalization strategies for the synthesis of a bifunctional dinucleotide.
Laura IANNAZZO received her PhD in 2010 at Pierre and Marie Curie University under the supervision of Dr C. AUBERT and Pr M. MALACRIA. She studied the reactivity of new unsaturated partners in cobalt-catalyzed [2+2+2] cycloaddition reactions. She then moved to the University of Pennsylvania for a post-doctorate with Pr G. MOLANDER. In 2012, she joined the UMR 8601 and the teams of Dr L. MICOUIN and Pr M. ETHEVE-QUELQUEJEU to work at the interface between chemistry and biology. Since 2016, she became CNRS Researcher at Paris University. Her research is focused on the development of methodologies for the synthesis of molecular tools based on the modification of RNAs and antibiotics.
Synthesis of a coordination complex under inert atmosphere

Photo taken by Erwan GALARDON
Coordination complexes are chemical entities in which one or several transition metals are bound to molecules called ligands. Because of the special electronic structures of transition metals, these complexes are often colored, their color being dependent on the cation and its coordinated ligands. This properties is for instance used to detect cations or analytes in various contexts (water analysis, biological imaging,…)
The synthesis of these complexes frequently involves water- or oxygen-sensitive intermediates, requiring the use of dedicated synthetic techniques. A standard technique is the “vaccum line”, allowing to carry out all steps of syntheses under an inert atmosphere (nitrogen or argon). In the experiment shown on the picture, a blue solution of a copper salt is added to a solution of ligand, leading to the formation of a red solution of complex. The full experimental set-up is placed under argon atmosphere using vaccum-argon cycles using three-ways stops cocks connected to both a vaccum and an argon line. The special glaswware (called “Schlenk tube”) with the copper salt solution is next placed under a higher pressure than the tube containing the ligand (using a needle seen on the picture), allowing the transfer of the solution with a metallic canula.
Erwan Galardon works for the CNRS and carries out his research in the Laboratoire de Chimie et Biochimie Pharmacologique et Toxicologie. His scientific interests lies in the synthesis and the study of the reactivity of coordination complexes with sulfur-containing ligands in a biological context, and he uses coordination chemistry to detect endogeneous sulfur-derivatives.
The first national nuclear magnetic resonance metabolomics platform dedicated to health (04/14/21) - MetaboParis-Santé

Photo taken by Gildas BERTHO (from left to right) : Microbiological safety station, Preparation robot, Sample changer, Spectrometer.
The first French platform to benefit from the IVDr (In Vitro Diagnostic research) system, MetaboParis-Santé is a reference center for obtaining metabolic profiles with a level of performance meeting international criteria.
The platform can analyze biological fluids, such as blood or urine, and obtain their molecular composition with unparalleled finesse, all in less than 15 minutes. This equipment is capable of processing 100 samples per day and is an important new step towards precision medicine. This technology is intended for research but also for personalized health monitoring. The analyzes carried out on the metabolic footprint present in the fluids of the human body will therefore make it possible to better understand a large number of diseases and to detect them well in advance, making this platform a major tool for public health monitoring.
Today, the system is used in research on various diseases for diagnosis with the identification of biomarkers, to make stratification, to follow the individual evolution over time, to better understand and to follow the effectiveness of new treatments, detect patients likely to develop complications in order to anticipate and improve their care.
Since 2012, the NMR research team has been developing an original metabolomic activity by NMR applied to the health field by offering a global vision of the metabolites produced by the human body or cells, to advance the understanding of the biological mechanisms of several diseases. and according to their treatment. The team has a panel of expertise covering all the crucial steps for the validation of an efficient analysis method at the interface of chemistry and biochemistry: sample preparation, spectroscopic development, development of an analytical method (targeted or not), biological interpretation. In this context, the team benefits from a network of scientific collaborations, in particular in the AP-HP hospitals, which makes it possible to develop a national project focused on health sciences.

Authors (from left to right) : Gildas Bertho (Ingénieur de recherche CNRS, directeur scientifique), Cédric Caradeuc (Assistant ingénieur – Université de Paris, responsable opérationnel), Nicolas Giraud (Professeur des universités, Université de Paris)
The extraction of an iron-catalysed reaction. An iron-ligand complex is the reason for the beautiful deep pink colour !

Photo taken by Hugo Esteves (M2 intern), Georgina Kirby (PhD student)
Our group aims to develop new sustainable methodologies to access complex molecules. We aim to do this by focusing on two key aspects of green chemistry: increasing atom economy and increasing step economy. To increase atom economy, we are using cheap and abundant iron as a catalyst to develop new reactions. For step economy, we wish to develop one-pot or domino procedures which ultimately means a reduced amount of waste is produced from reactions. Because nitrogen containing compounds are ubiquitous in many aspects of chemistry and in pharmaceutical compounds it is imperative that C-N bonds can be formed easily and sustainably.
Our group focuses on methodology, where we develop and optimise reactions. When performing an optimisation of a reaction, we aim to achieve the best results we can for the production of the desired product. We must repeat the reaction many times, usually only changing one parameter each time to see what effect, if any, it has on the outcome. The results from these experiments can give us an idea of how to proceed for the rest of the optimisation and can sometimes help elucidate how the reaction is occurring.
The photo shows an extraction which is performed after a reaction is completed. The reaction is washed with an aqueous solution where, in this case, the top layer is the aqueous phase, and the bottom layer is the organic containing our desired product solubilised in dichloromethane. After collection of the organic layer (bottom layer) and evaporation under reduced pressure, we have a crude product. We are very lucky to have recently been able to get a an automatic flash chromatography machine to purify compounds (figure 1), which helps make the process of developing reactions much faster and efficient!
When scientists think of iron chemistry they usually think of the same orange/brown colour (the rust colour!). Researchers who work with iron know that this is not always the case, as you can see from the photo, sometimes iron complexes can be pink, purple or red. This colour depends on the iron that you use as well as the ligand and solvent, where different combinations result in different colours. Because of these varying combinations, we see these changes in colour from experiment to experiment (figure 2).
Georgina Kirby is a 2nd year PhD student and Hugo Esteves is an incoming PhD student in the UMR8601 where they are both supervised by Guillaume Prestat and Farouk Berhal in the team ‘Organic Synthesis for biomedical research’ lead by Christine Gravier-Pelletier.
 |
 |
| Figure 1. Iron-Ligand complexes in solution. | Figure 2. Automatic Flash Chromatography Machine (BUCHI) |
Authors : Hugo Esteves (M2 intern), Georgina Kirby (PhD student)
Supervisors: Pr Guillaume Prestat and Dr Farouk Berhal
Macromolecular docking
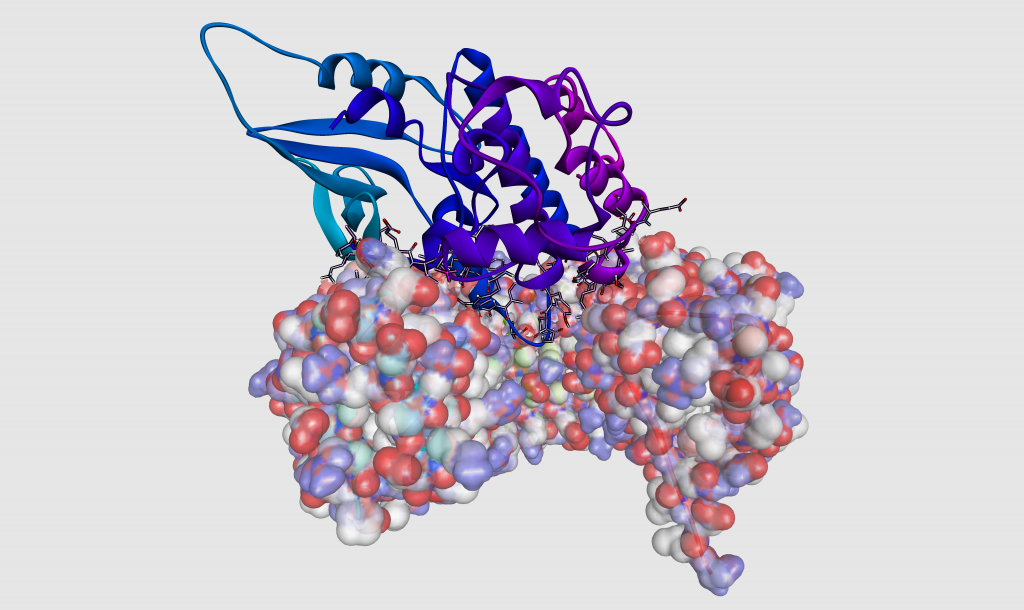
Photo taken by Laurent Le Corre
This image represents the 3D modeling of the interactions between two proteins. The molecule located above and represented as a ribbon, is a protein kinase of the cell cycle. The bottom molecule shown with its charged surface is the ribonuclease inhibitor protein. The positively and negatively charged regions are shown in blue and red, respectively. The amino acids located at the binding interface between the two proteins and more precisely in the anchoring loop are highlighted. This predictive model of the complex was obtained by the molecular docking technique from the crystallographic structures of each component. The calculation method is based on the complementarity of steric and physicochemical properties between the two partners.
The author
Laurent Le Corre studied chemistry at the University of Rennes and at the Ecole Nationale Supérieure de Chimie in Montpellier. Between 1998 and 2002, he worked successively at the Institute of Organic Chemistry in Lausanne, Switzerland then he joined the Laboratory of Natural Products and Environmental Chemistry at the University of Perpignan as a chemical engineer. Recruited at the CNRS in 2002 as a chemical synthesis engineer, he participated in the development of molecules of biological interest in the Organic Chemistry for Biomedical Research team and obtained his PhD degree in organic chemistry at the University of Paris Descartes in 2012. Since 2018, he is the operational manager of the Macromolecular Modeling Platform. His current activities include protein modeling, ligand–receptor interaction studies and virtual screening.
Synthon Z-D-Ala-D-Ala-OMe, a step towards the synthesis of “molecular fishing rods” for catching proteins
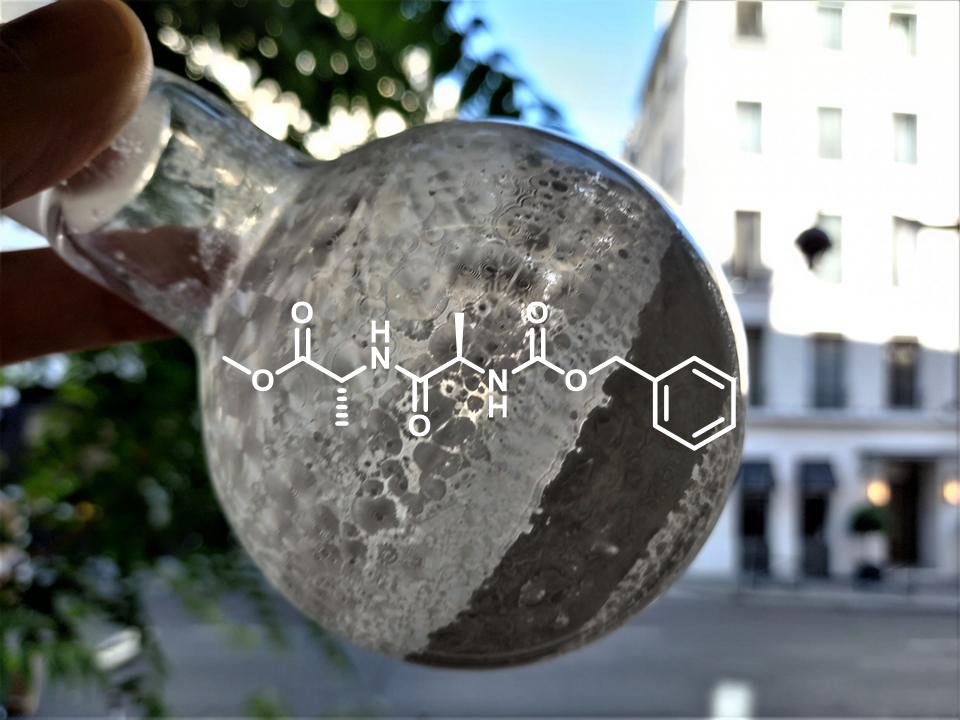
Photo taken by Michaël Bosco
Our laboratory is interested in the development of molecular probes to study rare diseases such as Congenital Disorders of Glycosylation (CDG). These diseases are characterized by the presence of proteins that are not correctly glycosylated. To study them, we are preparing molecular probes able to trap the enzymes responsible for these anomalies of glycosylations. These probes are made of three distinct parts: a first part mimicking the natural substrate of the enzyme, a middle part able to bind to the enzyme by a covalent bond and a last part allowing the extraction of the probe and enzyme assembly from biological media. The picture represents the last part of this probe. It consists of two D-Alanine amino acids linked by a peptide bond. It is the white product inside the flask resulting from a peptide coupling followed by a purification step by chromatography on silica gel.
Michaël Bosco, Post-doctoral fellow. My activities consist in the design, the synthesis and the analysis of these molecular probes.
The chemical library UMR8601 / PARIS DESCARTES department, a unifying project for the conservation of the laboratory's chemical heritage and its use on new biological targets.

Photo taken by Rodolphe Alves De Sousa
The chemical library UMR8601 / PARIS DESCARTES department has been part of the laboratories associated with the National chemical library (CN) of the CNRS since 2004 and with the chemical library pillar of ChemBioFrance since 2019. It is directed by Rodolphe Alves de Sousa, research engineer in chemical synthesis of CNRS since 2010. He was recruited at UMR8601 as a studies engineer in 1999, was appointed prevention assistant in 2005, defended his thesis at the doctoral school of drugs in 2009, member of the CN steering committee since 2018 and is co-author of 23 publications and 2 patents.
The chemical library is a kind of library where a large part of the laboratory’s chemical synthesis work is stored. This reserve of products of interest is made up of nearly 6,000 compounds from both old collections found in the cupboards and more recent products on current themes, thus making it possible to cover a great structural and thematic diversity. We have set up a protocol (sorting – identification – analyzes and storage) which makes it possible to manage the synthetic products (intermediates and final) of the last 30 years for completed, terminated or abandoned subjects. All the structures are integrated into an database common to the entire laboratory with a view to exploiting these compounds for other applications, whether by virtual screening or physical screening. These compounds, depending on the quantities available and the agreement of the chemists, can also serve as a building block for further syntheses. Go therefore consult the database to find out if your product or analogues have already been synthesized in the laboratory.
https://lcbpt.biomedicale.parisdescartes.fr/chemical-library/
Electron paramagnetic resonance detection of a nanoformulated nitroxide radical in the head of an anesthetized mouse, after tail vein injection.
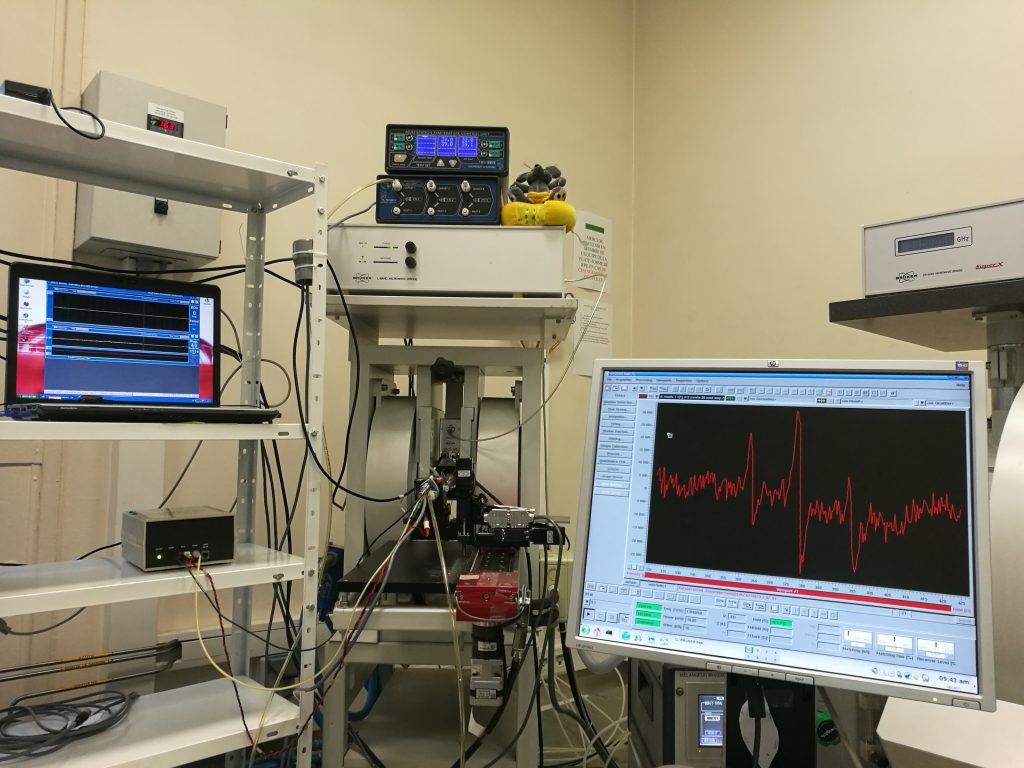
Photo taken by Sonia Lajnef & Fabienne Peyrot
Electron paramagnetic resonance (EPR) is the state-of-the-art method to detect paramagnetic species. This technique, in association with the use of radical probes, is considered promising to monitor the variations of redox homeostasis in vivo in a relatively direct and minimaly invasive manner. However, several technological drawbacks prevent its development, mainly linked to spectrometers, signal acquisition and processing methods, and spin probes. We focus on this last aspect by developing innovative probes with enhanced stability, derived from aminoxyl (nitroxide) radicals with an isoindoline core substituted by four ethyl groups.
At the center of the picture, the EPR spectrometer-imager of UMR 8601, operating with microwave radiations at a frequency of 1.2 GHz, can be identified from its two magnet coils. Just before EPR analysis, a nitroxide radical synthesized in the lab and formulated in an oil-in-water nanoemulsion in collaboration with Caroline Roques (UTCBS, Faculty of Pharmacy) has been injected in the tail vein of a normal mouse. The head of the sleeping mouse is positioned inside the resonating cavity in the air gap of the magnet. The mouse is kept under isoflurane gaseous anesthesia throughout the experiment. Its breathing rhythm and its body temperature are constantly monitored on the left screen. The EPR signal recorded from the head region is displayed on the screen at the right. The three-line EPR spectrum is characteristic of a nitroxide radical in which the electron spin is coupled to the nuclear spin of nitrogen-14. Preliminary results show a time-dependent signal decay with an apparent half-life of 23 min. This is very encouraging as reported half-lives for commercially available tetramethyl-substituted probes range between 3 and 10 min.
Sonia Lajnef is a CNRS engineer and operational manager of the EPR platform since 2011. Fabienne Peyrot is an associate professor at Institut National Supérieur du Professorat et de l’Éducation (Sorbonne University) who joined UMR 8601 in 2006. They are both members of the team “Redox homeostasis and bio(in)organic chemistry of reactive sulfur species” directed by Dominique Padovani.
This experiment could not have been carried out without the help of Claire Mader, head of the animal facility at UFR des Sciences Fondamentales et Biomédicales.
