Riccardo PICCARDI
Research Scientist, CNRS

Laboratoire de Chimie et Biochimie pharmacologiques et toxicologiques (LCBPT) – CNRS UMR 8601
Université Paris Descartes
45 Rue des Saints Pères
75270 Paris Cedex 06
France

Riccardo PICCARDI
Research Scientist, CNRS
Chemistry towards life sciences
Pharmacologiques et Toxicologiques (LCBPT)
CNRS UMR 8601
Université Paris Descartes
45 Rue des Saints Pères
75270 Paris Cedex 06
France
Curriculum Vitae
Academic and Professional Background
| 2014- | CNRS Researcher. UMR 8601, University of Paris Descartes. |
| 2009- 2014 | CNRS Researcher. UMR 8182, University of Paris Sud. |
| 2008-2009 | Temporary Associate Professor (ATER). UMR 8182, University of Paris Sud. |
| 2006-2008 | Post-Doctoral Fellow University of Claude Bernard Lyon I (with Prof. O. Baudoin) |
| 2005-2006 | Post-Doctoral Fellow Technical University of Munich (TUM)-Germany (with Prof. T. Bach) |
| 2001-2005 | Ph.D. in chemistry. University of Bern-Switzerland (with Prof. P. Renaud) |
| 2000-2001 | Master in Chemistry (Laurea). University of Florence -Italy (with Dr. G. Reginato) |
Fundings:
| 2024 | IdEX “Emergence” Université paris Cité (projet Light Carbenes) |  |
| 2010 | “Atractivité ” Université Paris Sud-11 |
Réseaux
GdR Synthflux
Collaborations:
Pr. Emmanuelle SACHON (Laboratoire de Biomolécules -Sorbonne Université -ENS)
Teaching
– Introduction to flow chemistry (2h intervention during Green Chemistry teaching unit)
– “Travaux Dirigés” LASS (minor chemistry)
Administratives tasks
– Health and safety assistant
– Webmaster
Languages
French
ENglish
Italian
Research
The reserch I am developing is focused on two main subjects:
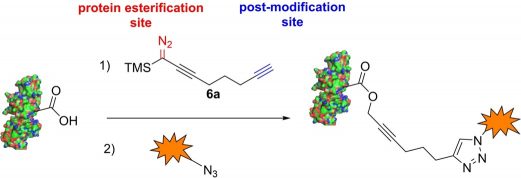
– the reactivity of α-alkynyl-diazosilanes: a very interesting new class of compounds that can react easily with carboxylic acids and can be also used in the functionalisation of proteins in biological media.
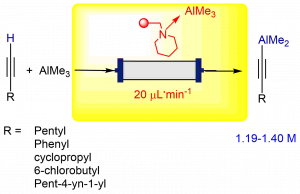
– the reactivity of dimethylalkynylaluminum reagents and continuous flow reactions
Publications
2025
- R. Piccardi, L. Micouin, “An Overview on the direct preparation of Organoaluminum compounds” 2025, accepted, DOI. 10.1055/a-2669-5996
2024
- T. Zhao, E. Sachon, L. Micouin, R. Piccardi “α-Silylated Diazoalkynes: New Tools for Bioconjugation of Proteins” 2024, 30, e202302807.
DOI: 10.1002/chem.202302807 - L. Micouin, R. Piccardi, Organoaluminum Reagents, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2024, ISBN 9780124095472, DOI: 10.1016/B978-0-323-96025-0.00041-7.
2019
- Zhao, L. Micouin, R. Piccardi. “Synthesis of Organometallic Compounds in Flow”. Helv. Chim. Acta, 2019, ⟨10.1002/hlca.201900172⟩.
2018
- Piccardi, S. Turcaud, E. Benedetti, L. Micouin. “Synthesis and Reactivity of Mixed Dimethylalkynylaluminum Reagents”. Synthesis, 2018, 51, 97-106. ⟨10.1055/s-0037-1610392⟩.
- Zhao, R. Piccardi, L. Micouin. “Rapid and Effective Synthesis of α-Acyloxy-α-alkynyltrimethylsilanes”. Org. Lett. 2018, 20, 5015-5018. ⟨10.1021/acs.orglett.8b02165⟩.
2016
- R. Piccardi, A. Coffinet, E. Benedetti, S. Turcaud, L. Micouin, “Continuous flow synthesis of dimethylalkynylaluminum reagents” Synthesis 2016, 48, 3272-3278
- R. Piccardi, L. Micouin, O. Jackowski “The Chemistry of Alkynylaluminum Species”. I. Marek Ed. PATAI’S Chemistry of Functional Groups, John Wiley & Sons, Ltd, 1-47, 2016, DOI: https://doi.org/10.1002/9780470682531.pat0832
Older publications
- Prosa, R. Turgis, R. Piccardi, M.-C. Scherrmann, “Soluble Polymer-Supported Flow Synthesis: A Green Process for the Preparation of Heterocycles” Eur. J. Org. Chem. 2012, 2188-2200
- Chaumontet, R. Piccardi, O. Baudoin “Synthesis of 3,4-Dihydroisoquinolines by a C(sp3)–H Activation-Electrocyclization Strategy: Total Synthesis of Coralydine” Angew. Chem. Int. Ed. 2009, 48, 179-182
- Chaumontet, R. Piccardi, N. Audic, J. Hitce, J.-L. Peglion, E. Clot, O. Baudoin, “Synthesis of Benzocyclobutenes by Palladium-Catalyzed C−H Activation of Methyl Groups: Method and Mechanistic Study” J. Am. Chem. Soc. 2008, 130, 15157 – 15166
- Piccardi, P. Renaud, “Formal Synthesis of (+) and (-)-Ferruginine” Eur. J. Org. Chem. 2007, 10, 4752-4757.
- Reginato, A. Mordini, M. Valacchi, R. Piccardi “Synthesis of non-racemic b-branched a-(aminoalkyl)-acrylates from naturally occurring amino acids” Tetrahedron: Asymmetry 2002, 13, 595-600