
Pharmacologiques et Toxicologiques (LCBPT)
CNRS UMR 8601
Université Paris Descartes
45 Rue des Saints Pères
75270 Paris Cedex 06
France
Guillaume PRESTAT
Professor Pr1

Laboratoire de Chimie et Biochimie pharmacologiques et toxicologiques (LCBPT) – CNRS UMR 8601
Université Paris Descartes
45 Rue des Saints Pères
75270 Paris Cedex 06
France
Curriculum Vitae
- Membre CA Université de Paris
- Responsable enseignement de chimie UFR Sciences Fondamentales et Biomédicales
- Directeur service TP Chimie de la faculté des sciences fondamentales et biomédicales
- Membre de la Societé Chimique de France
- 2011 – Professeur (section 32) – Université Paris Descartes
- 2010 – Chaire Actelion en chimie organique innovante
- 2006 – Habilitation à diriger des recherches, Université Paris VI -Cyclisations catalysées par le palladium & enchaînements domino.
- 2000 – Maître de Conférences – Université Pierre et Marie Curie Paris VI – IPCM – Equipe Pr. Giovanni Poli
- 1998-2000 – Stage Post-Doctoral – Synthèse d’acétogénines par réaction de métathèse – Dr Charles Mioskowski CEA Saclay – Service des Molécules Marquées.
- 1997-98 – ATER Faculté des sciences et technique de l’Université de Nantes 1998 – Thèse de Doctorat : Synthèse d’analogues thiodésoxynucléosidiques – Dr Jean-Paul Pradère
Research
We are developing new synthetic methodologies based on the concepts of atom and step economy. To this goal, we design original transition-metal-catalyzed domino processes. The methods developed allow the access to privileged structures in medicinal chemistry. We recently developed a green process based on the iron catalyzed generation of nitrene to perform the aziridination of alkenes.
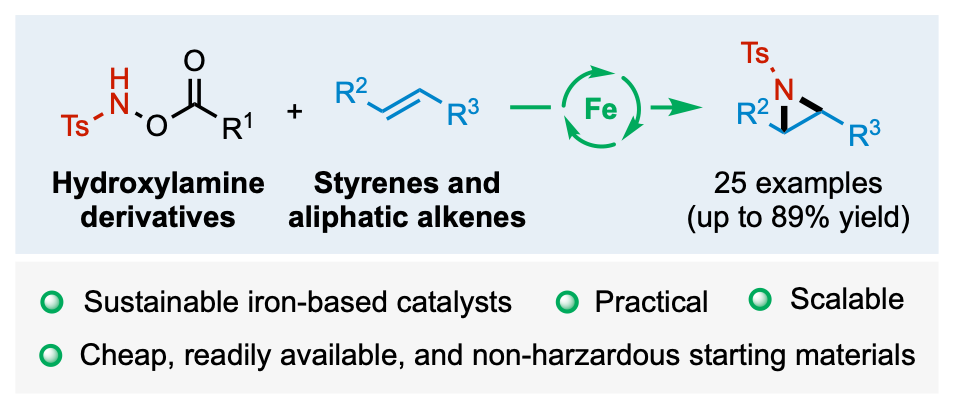
Kirby, G.; Grimaud, L.; Vitale, M. R.; Prestat, G.; Berhal, F. Iron(II)-Catalyzed Intermolecular Aziridination of Alkenes Employing Hydroxylamine Derivatives as Clean Nitrene Sources. Green Chem. 2021, 23 (23), 9428–9432. https://doi.org/10.1039/D1GC03495B.
Publications
Book Chapters




1) Kammerer-Pentier, C.; Liron, F.; Prestat, G.; Poli, G, “Selectivity in Palladium Catalyzed Allylic Substitution” in Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis (Kazmaier U. Ed), Topics in Organometallics Chemistry 38, pp 1-64, Springer-Verlag Berlin and Heidelberg GmbH & Co.
2) Avers, M.C.; Bonaccorsi, P.; Madec, D.; Prestat, G.; Poli, G, The Fabulous Destiny of Sulfenic Acids in Innovative Catalysis in Organic Synthesis: Oxidation, Hydrogenation, and C-X Bond Forming Reactions (Andersson, P. G. Ed), pp 47-103, Wiley-VCH Verlag GmbH & Co. KGaA
3) Lorion, M. M. ; Kammerer, C. ; Prestat, G. ; Poli, G., Sequential Pd-catalyzed allylic alkylation / Ru-catalyzed ring-closing metathesis in Comprehensive Organic Chemistry Experiments for the Laboratory Classroom, Eds. Afonso, C. A. M. ; Candeias, N. R. ; Simão, D. P. ; Trindade, A. F. ; Coelho, J. A. S. ; Tan, B. ; Franzén R., The Royal Society of Chemistry, 2017, 5, 650-655.
4) Fichez, J.; Busca, P.; Prestat, G. Recent Advances In Aminopyrazoles Synthesis And Functionalization in Targets in Heterocyclic Systems, ed. O. A. Attanasi, P Merino and D. Spinelli, Italian Society of Chemistry, Roma, 2017, vol. 21, pp. 322-347
2024
Esteves, H.; Xavier, T.; Lajnef, S.; Peyrot, F.; Lefèvre, G.; Prestat, G.*; Berhal, F.* “Iron-Catalyzed Intramolecular C(sp3)-H Lactonization of Hydroxamate Derivatives Promoted by a 1,5-HAT.” ACS Catal. 2024, 14 (6), 4329–4339. https://doi.org/10.1021/acscatal.3c04900.
2023
2022
da Cunha, M. F.; Pranke, I.; Sassi, A.; Schreiweis, C.; Moriceau, S.; Vidovic, D.; Hatton, A.; Carlon, M. S.; Creste, G.; Berhal, F.; Prestat, G.; Freund, R.; Odolczyk, N.; Jais, J. P.; Gravier-Pelletier, C.; Zielenkiewicz, P.; Jullien, V.; Hinzpeter, A.; Oury, F.; Edelman, A.; Sermet-Gaudelus, I. Systemic Bis-Phosphinic Acid Derivative Restores Chloride Transport in Cystic Fibrosis Mice. Sci Rep 2022, 12 (1), 6132. https://doi.org/10.1038/s41598-022-09678-9.
2021
Kirby, G.; Grimaud, L.; Vitale, M. R.; Prestat, G.; Berhal, F. Iron(II)-Catalyzed Intermolecular Aziridination of Alkenes Employing Hydroxylamine Derivatives as Clean Nitrene Sources. Green Chem. 2021, 23 (23), 9428–9432 doi: 10.1039/D1GC03495B
Bitam, S.; Elbahnsi, A.; Creste, G.; Pranke, I.; Chevalier, B.; Berhal, F.; Hoffmann, B.; Servel, N.; Tondelier, D.; Hatton, A.; Moquereau, C.; Faria Da Cunha, M.; Pastor, A.; Lepissier, A.; Hinzpeter, A.; Mornon, J.-P.; Prestat, G.; Edelman, A.; Callebaut, I.; Gravier-Pelletier, C.; Sermet-Gaudelus, I. New Insights into Structure and Function of Bis-Phosphinic Acid Derivatives and Implications for CFTR Modulation. Sci Rep 2021, 11 (1), 6842. https://doi.org/10.1038/s41598-021-83240-x.
Bardin, E.; Pastor, A.; Semeraro, M.; Golec, A.; Hayes, K.; Chevalier, B.; Berhal, F.; Prestat, G.; Hinzpeter, A.; Gravier-Pelletier, C.; Pranke, I.; Sermet-Gaudelus, I. Modulators of CFTR. Updates on Clinical Development and Future Directions. Eur. J. Med. Chem. 2021, 113195 doi.org/10.1016/j.ejmech.2021.113195
2020
- Abi Fayssal, S.; Giungi, A.; Berhal, F.; Prestat, G. Iron-Catalyzed Intra-Intermolecular Aminoazidation of Alkenes. Org. Process Res. Dev. 2020, 24, 695–703 10.1021/acs.oprd.9b00400.
- Fichez, J.; Soulie, C.; Corre, L. L.; Sayon, S.; Priet, S.; Alvarez, K.; Delelis, O.; Gizzi, P.; Prestat, G.; Gravier-Pelletier, C.; Marcelin, A.-G.; Calvez, V.; Busca, P. Discovery, SAR Study and ADME Properties of Methyl 4-Amino-3-Cyano-1-(2-Benzyloxyphenyl)-1H-Pyrazole-5-Carboxylate as an HIV-1 Replication Inhibitor. RSC Med. Chem. 2020. asap doi.org:10.1039/D0MD00025F.
2019
2018
- Manick, A.-D.; Aubert, S.; Yalcouye, B.; Prangé, T.; Berhal, F.; Prestat, G. Access to functionalized imidazolidin‐2‐one derivatives by iron‐catalyzed oxyamination of alkenes, Chem. Eur. J. 2018, 24, 11485-11492. 10.1002/chem.201802190
- Fichez, J.; Prestat, G.; Busca, P. Reductive Cleavage of Aromatic and Heteroaromatic Ester Functions via Copper-Catalyzed Proto-Decarbomethoxylation, Org. Lett. 2018, 48, 2724-2727. 10.1021/acs.orglett.8b00930
- Manick, A.-D.; Berhal, F.; Prestat, G. Development of a One-Pot Four C−C Bond-Forming Sequence Based on Palladium/Ruthenium Tandem Catalysis, Org. Lett. 2018, 48, 194-197. 10.1021/acs.orglett.7b03556
2017
- Mao, Z.; Martini, E; Prestat, G.; Oble, J.; Huang, P.-Q.; Poli, G.; Analogues of the 2-carboxyl-6-hydroxyoctahydroindole (CHOI) unit from diverging Pd-catalyzed allylations: Selectivity as a function of the double bond position, Tetrahedron Lett. 2017, 58, 4174-4178. 10.1016/j.tetlet.2017.09.046
