Supramolecular Bio-inorganic Chemistry
Team Composition
Permanent Staff
Benoît Colasson, Pr (Group leader)
Benjamin Doistau, MCU, (Ass. Prof.)
PhD Students
Bilel Gherdaoui
Master students
Colin Zeinoulabadine

Context: In the biological world, proteins act as highly efficient and selective receptors. Recognition processes are based on multiple non-covalent interactions. However, many proteins also contain metal ions that can be directly involved in recognition processes or even act as a catalyst leading to the selective transformation of an organic substrate. In enzymes, the control of the metallo-site is due to the structuring of the protein backbone in order to not only preorganize the coordination site for the metal ion, but also provide a cavity and a corridor connecting it to the bulk. All these features lead to remarkable control of the binding of exogenous molecules and the reactivity of the metal ion.

Interest: We are interested in developing supramolecular models that will allow the control of the first and the second coordination spheres as well as the substrate access channel that selects and drives the substrate to the metal center, and expels the product. Our strategies consist in:
i) The covalent linkage of a coordination site, which will reproduce the natural core provided by the amino acid residues found in the enzymes, to a hydrophobic cavity that will protect the metal center and play the role of the enzyme active site pocket;
ii) The encapsulation of simple biomimetic metal complexes within a supramolecular cage.
Goals:
- Get key information on Nature’s strategy for controlling the reactivity of a metal ion confined in an organic pocket (active site of enzymes).
- Design molecular receptors and redox probes.
- Discover new reactive pathways by constraining in a well-defined cavity space the catalyst (metal ion), the reactant (small molecules such as H2O, O2, CO2) and guest substrate.
- Obtain selective and efficient supramolecular catalysts.
- Control other metal complexes’ properties (e.g. luminescence)
Key-words: Biomimetic metal complexes, Host-guest chemistry, Chirality.
Expertise: Supramolecular Chemistry, Coordination Chemistry, Biomimetic Chemistry, Macrocyclic chemistry, Calixarenes and Resorcinarenes.
Tools: Organic synthesis (functionalization of molecular hosts, synthesis of ligands); coordination chemistry of Zn, Cu, Cr; Spectroscopies (NMR, EPR, UV-vis-NIR, fluorescence, IR), mass spectrometry; cyclic voltammetry…
Supramolecular Bio-inorganic Chemistry
Team composition
Permanent Staff
Benoît Colasson, Pr (Group leader)
Benjamin Doistau, MCU, (Ass. Prof.)
PhD Students
Bilel Gherdaoui
Master students
Colin Zeinaoulabadine (M2)
Team Description

Context: In the biological world, proteins act as highly efficient and selective receptors. Recognition processes are based on multiple non-covalent interactions. However, many proteins also contain metal ions that can be directly involved in recognition processes or even act as a catalyst leading to the selective transformation of an organic substrate. In enzymes, the control of the metallo-site is due to the structuring of the protein backbone in order to not only preorganize the coordination site for the metal ion, but also provide a cavity and a corridor connecting it to the bulk. All these features lead to remarkable control of the binding of exogenous molecules and the reactivity of the metal ion.

Interest: We are interested in developing supramolecular models that will allow the control of the first and the second coordination spheres as well as the substrate access channel that selects and drives the substrate to the metal center, and expels the product. Our strategies consist in:
i) The covalent linkage of a coordination site, which will reproduce the natural core provided by the amino acid residues found in the enzymes, to a hydrophobic cavity that will protect the metal center and play the role of the enzyme active site pocket;
ii) The encapsulation of simple biomimetic metal complexes within a supramolecular cage.
Goals:
- Get key information on Nature’s strategy for controlling the reactivity of a metal ion confined in an organic pocket (active site of enzymes).
- Design molecular receptors and redox probes.
- Discover new reactive pathways by constraining in a well-defined cavity space the catalyst (metal ion), the reactant (small molecules such as H2O, O2, CO2) and guest substrate.
- Obtain selective and efficient supramolecular catalysts.
- Control other metal complexes’ properties (e.g. luminescence)

Key-words: Biomimetic metal complexes, Host-guest chemistry, Chirality.
Expertise: Supramolecular Chemistry, Coordination Chemistry, Biomimetic Chemistry, Macrocyclic chemistry, Calixarenes and Resorcinarenes.
Tools: Organic synthesis (functionalization of molecular hosts, synthesis of ligands); coordination chemistry of Zn, Cu, Cr; Spectroscopies (NMR, EPR, UV-vis-NIR, fluorescence, IR), mass spectrometry; cyclic voltammetry…
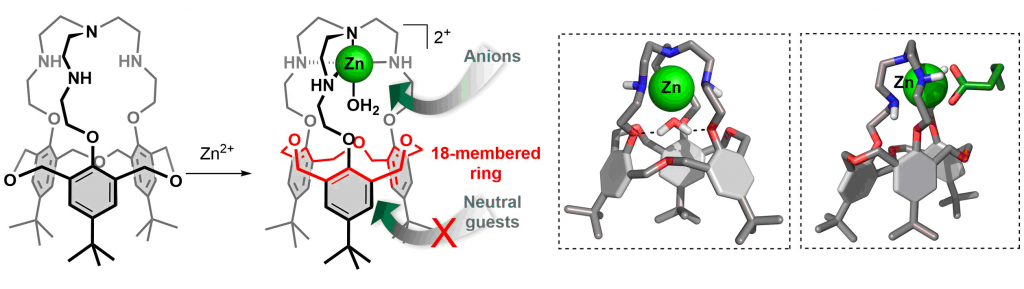
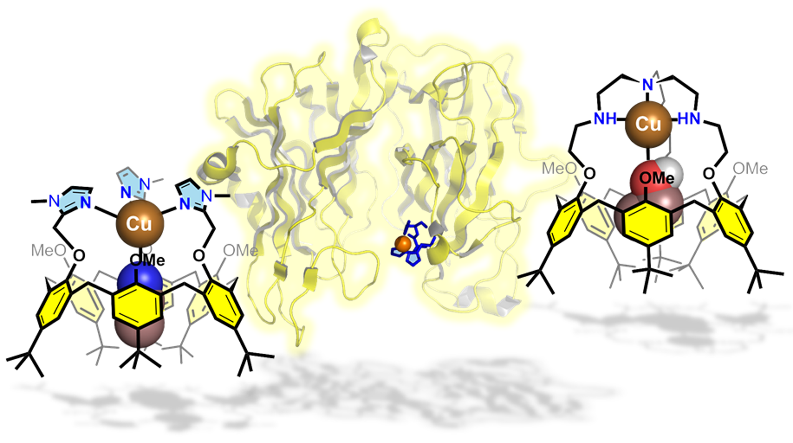
“Supramolecular Modeling of Mono-Copper Enzyme Active Sites with Funnel-Complexes”, Le Poul, Y. Le Mest, I. Jabin, O Reinaud, Acc. Chem. Res., 2015, 48, 2097–2106. DOI: 10.1021/acs.accounts.5b00152.
“Supramolecular Control of Transition Metal Complexes in Water by a Hydrophobic Cavity: a Bio-Inspired Strategy”, Bistri and O. Reinaud,* Org. Biomol. Chem., 2015, 13, 2849–2865. DOI: 10.1039/C4OB02511C.
“Biomimetic Cavity-Based Metal Complexes”, J.-N. Rebilly, B. Colasson, O. Bistri, D. Over, O. Reinaud,* Chem. Soc. Rev. 2015, 44, 467-489. DOI: 10.1039/c4cs00211c.
“Calixarenes and resorcinarenes as scaffolds for supramolecular metallo-enzyme mimicry”, J.-N. Rebilly, O. Reinaud, Supramol. Chem. (special issue “calix13”), 2014, 26, 454-479. DOI: 10.1080/10610278.2013.877137.
“Biomimetic and self-assembled calix[6]arene-based receptors for neutral molecules”, Coquière, S. Le Gac, U. Darbost, O. Sénèque, I. Jabin, O. Reinaud, Org. Biomol. Chem., 2009, 7, 2485-2500 (Perspective).
“Synthesis and Binding Properties of a Tren-Capped Hexahomotrioxacalix[3]arene”, S. Zahim, D. Ajami, P. Laurent, H. Valkenier, O. Reinaud, M. Luhmer, I. Jabin*, Chem. Phys. Chem. 2020. DOI: 10.1002/cphc.201900951.