Class C G Protein Coupled Receptors (GPCRs)
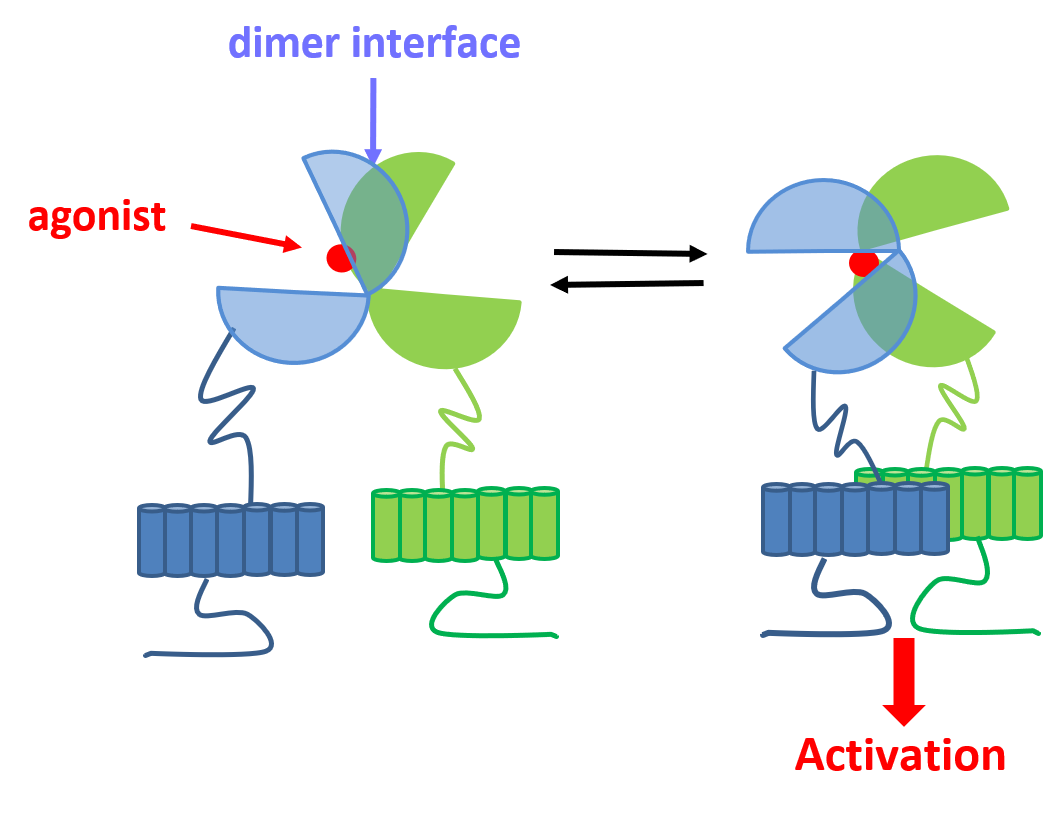
GPCRs make up the largest family of small molecule targets on the drug market. However these mostly belong to class A, although class C receptors are perceived as highly valuable new targets. Among these, metabotropic glutamate receptors (mGluRs) are found. Glutamate is the major excitatory neurotransmitter in the brain. It activates ligand gated ion channels (ionotropic receptors, iGluRs) and mGluRs. The iGluRs are responsible of the fast synaptic transmission while mGluRs are modulators of that event. Accordingly, mGluRs may allow a fine-tuning that is required for CNS treatments. Class C GPCRs are characterized by a large amino terminal domain that folds in two lobes connected by a flexible hinge and named Venus Flytrap (VFT) domain. The agonist binding site is located in the cleft near the hinge. It has been demonstrated that the VFT domain needs to adopt a closed conformation that induces a movement of the receptor dimer in order to trigger activation and signal transduction.
A.
B.
C.
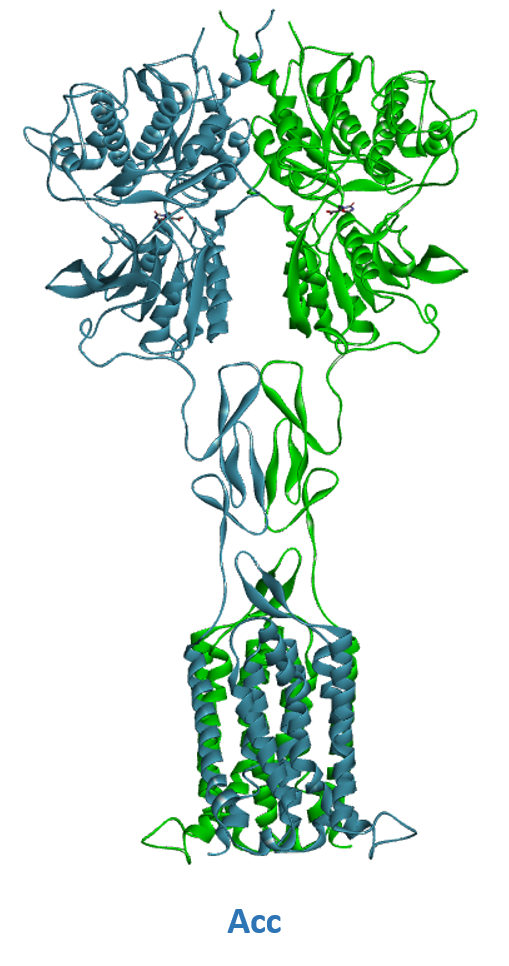
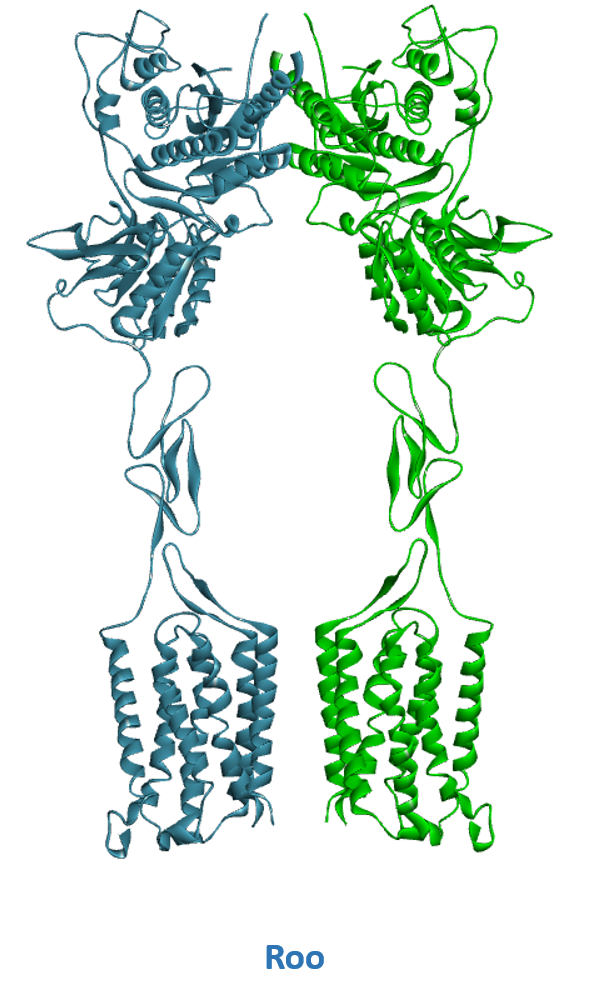
- A) Schematic representation of Class C GPCR’s activation mechanism. B) mGluR in its resting conformation with open VFTs and 7TM domains apart (Roo). C) mGluR in its active conformation with closed VFTs, bound agonist and 7TM domains in contact (Acc).
Eight subtypes of mGluRs have been identified and classified in three groups (mGlu1-5 in group-I, mGlu2-3 in group-II and mGlu4-6-7-8 in group-III). Besides mGlu6 that is only present in the retina, group-III receptors are mostly presynaptic. Their activation will reduce the release of glutamate or GABA from hetero-receptors. We have been involved for many years in the development of group-III receptor orthosteric ligands and in activation mechanism investigation. We initially built a homology model of the VFT domain of mGlu4 docked L-AP4, a phosphonate analogue of glutamate that selectively activates group-III mGlu receptors. We then ran a virtual high through screening (vHTS) of the binding domain and discovered PCEP that was optimized in a series of analogues. PCEP allowed us to disclose a new binding pocket next to the glutamate binding site where in fact a chloride is bound. This new pocket is lined with selective residues which explain the mGlu4 selectivity of LSP4-2022 and allowed the design of other subtype selective orthosteric agonists. Later we discovered a second chloride site adjacent to the glutamate binding site and where LSP4-2022 may be binding.
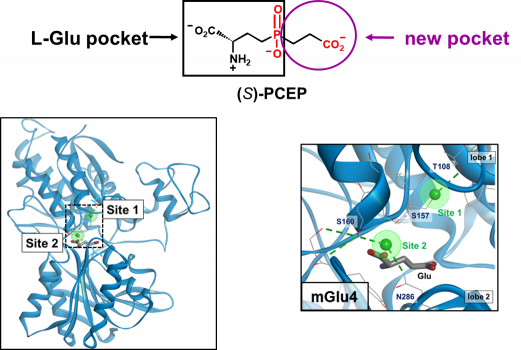
The two chloride binding sites of the mGlu4 VFT domain
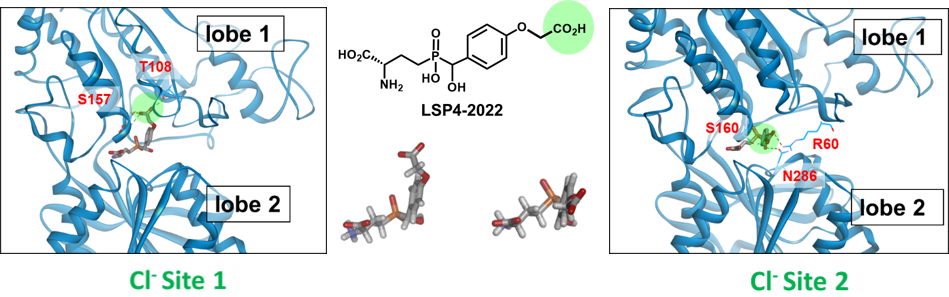
LSP4-2022 can bind to the two chloride sites when adopting two different conformations.
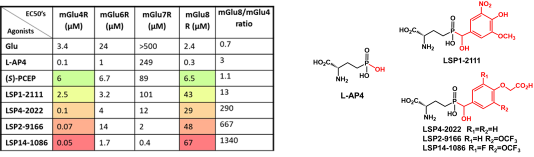
Since the discovery of PCEP, more than 150 derivatives have been synthesized and tested in vitro gaining potency and selectivity (see Table below).
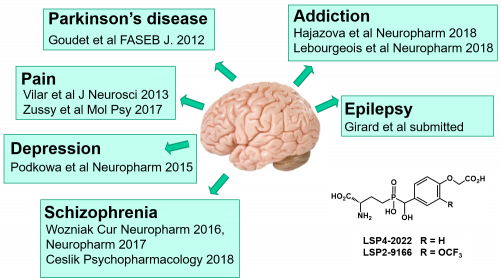
Some of these group-III selective agonists have been evaluated in preclinical studies for CNS pathologies. They all show beneficial effects and advantageous properties such as high aqueous solubility, brain penetration and metabolic stability. Most recent studies are recapitulated below.
We are currently pursuing the development of mGlu4 and mGlu7 selective ligands with the help of 3D-models.
